Cgmp Course
Cgmp Course - Food manufacturing for professionals (21 cfr part 117) subcategories (areas of specialty or focus): Teaching employees how to identify, assess, and mitigate risks. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. Current good manufacturing practice (cgmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. In this module, we will learn about advanced concepts in supply chain management. Web the full length course includes an introduction to current good manufacturing practices (cgmps), information on maintaining product quality, the scope of gmp rules, a section on cleaning and sanitation, a look into proper documentation, and concludes with a. Ensuring personnel understand the importance of validation in maintaining product quality. Web cgmp compliance:popular programs & training categories. Web cgmp refers to the current good manufacturing practice regulations enforced by the fda. The registration fee for each course. Providing training on validation processes and equipment qualification. (2 or 4 credit hours) Cgmp training course is designed for anyone who needs a good understanding of current good manufacturing practices. Module 5 • 3 hours to complete. Web cgmp compliance:popular programs & training categories. Web cgmp refers to the current good manufacturing practice regulations enforced by the fda. Web $23,904 usd tuition total tuition for other mba programs can easily exceed $200,000. Individuals who have earned their cgmp have obtained the highest designation available that is specifically for government meeting. The registration fee for each course. It is designed to minimize the risks involved. It covers key principles of the who and pic/s standards and will equip participants with a broad, foundational understanding of gmp. Explaining risk assessment and management processes within cgmp. First, you will learn about network planning in supply chains, and supply chain network design. With the imba, you'll save substantially on your total cost while pursuing an mba from a. In this module, we will learn about advanced concepts in supply chain management. Fundamental courses for drug manufacturers. See individual course pages for. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. With the imba, you'll save substantially on your total cost while pursuing an mba from a leading. First, you will learn about network planning in supply chains, and supply chain network design. Web the certified pharmaceutical gmp professional understands the good manufacturing practices (gmp) as regulated and guided by national and international agencies for the pharmaceutical industry. Web $23,904 usd tuition total tuition for other mba programs can easily exceed $200,000. Prepare for the gmpro™ certification at. Gmp basic course (in english): Providing training on validation processes and equipment qualification. Web ( 21 reviews) download price: Web this free online course provides an overview of essential gmp principles and requirements. Current good manufacturing practice (cgmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Web the certified government meeting professional designation (cgmp) is designed for planners and suppliers whose work is governed by the rules and regulations of the federal government. Web the certified pharmaceutical gmp professional understands the good manufacturing practices (gmp) as regulated and guided by national and international agencies for the pharmaceutical industry. Individuals who have earned their cgmp have obtained. Explaining risk assessment and management processes within cgmp. Teaching employees how to identify, assess, and mitigate risks. Whether it is solo learning or group learning, we have many years of experience in managing training projects of any size. Web $23,904 usd tuition total tuition for other mba programs can easily exceed $200,000. Introduction to current good manufacturing practices (cgmps) maintaining. Web this free online course provides an overview of essential gmp principles and requirements. Food manufacturing for professionals (21 cfr part 117) subcategories (areas of specialty or focus): This course provides an intensive conceptual and applied introduction to auditing in society. Web each cgmp certification training course is designed to present and explain cgmp mandates, as well as to provide. This covers finished human and veterinary drugs and biologics, ectoparasiticides, and dietary supplements (alternatively called nutraceuticals) where. It covers key principles of the who and pic/s standards and will equip participants with a broad, foundational understanding of gmp. In this module, we will learn about advanced concepts in supply chain management. Introduction to good manufacturing practice (gmp) course includes sections. Introduction to current good manufacturing practices (cgmps) maintaining product quality scope of gmp rules cleaning and sanitation proper documentation summary. Web each cgmp certification training course is designed to present and explain cgmp mandates, as well as to provide comprehensive analysis and instruction on how to best comply. Whether it is solo learning or group learning, we have many years of experience in managing training projects of any size. Each year, estimates suggest that 1/3 of all food produced is lost or wasted, making postharvest loss. Teaching employees how to identify, assess, and mitigate risks. It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing. Cgmp training course is designed for anyone who needs a good understanding of current good manufacturing practices. This course provides an intensive conceptual and applied introduction to auditing in society. It is designed to minimize the risks involved in any production that cannot be eliminated through testing the final product. To view our complete cgmp training course catalog, click here. It covers key principles of the who and pic/s standards and will equip participants with a broad, foundational understanding of gmp. Providing training on validation processes and equipment qualification. Individuals who have earned their cgmp have obtained the highest designation available that is specifically for government meeting. This course provides an overview of the issue of postharvest loss of grains by exploring essential physical, technical, and social dimensions of postharvest supply chains and loss prevention methods globally. Ensuring personnel understand the importance of validation in maintaining product quality. Web cgmp refers to the current good manufacturing practice regulations enforced by the fda.
Qualstar Simulation An Advanced CGMP Training Course The Health

cGMP Training, Current Good Manufacturing Practices, FDA cGMP, QSR and

Introduction to Good Manufacturing Practices (cGMP) Training
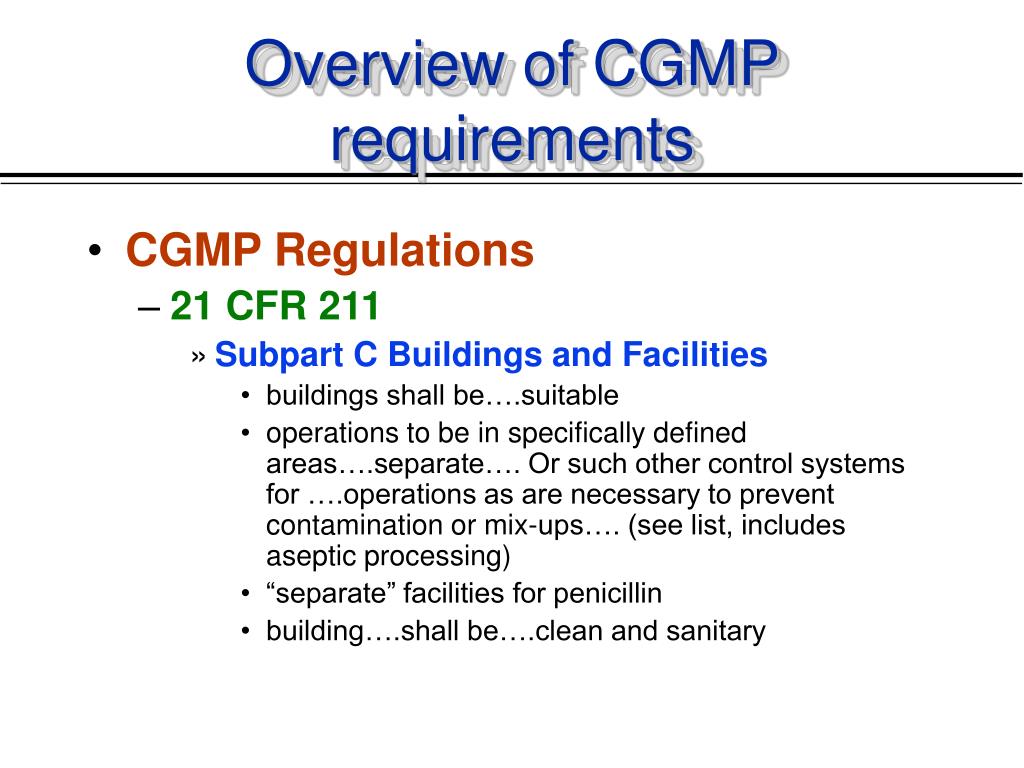
PPT FDA cGMP Training Program PowerPoint Presentation, free download

cGMP Training 2013

Advanced cGMP Training For Leaders & Top Management The Health, Drug

GMP Olympics cGMP Training The Health, Drug, Prescription, and GMP

PPT FDA cGMP Training Program PowerPoint Presentation, free download
Introduction To CGMP Compliance Course PDF Tablet (Pharmacy

PPT FDA cGMP Training Program PowerPoint Presentation, free download
Web The Certified Pharmaceutical Gmp Professional Understands The Good Manufacturing Practices (Gmp) As Regulated And Guided By National And International Agencies For The Pharmaceutical Industry.
This Accredited Online Training Course Will Teach You About Good Manufacturing Practice (Cgmp) Which Is A System For Ensuring That Products Are Consistently Produced And Controlled According To Quality Standards.
First, You Will Learn About Network Planning In Supply Chains, And Supply Chain Network Design.
Explaining Risk Assessment And Management Processes Within Cgmp.
Related Post:
