Computer System Validation Courses
Computer System Validation Courses - Web this course covers the principles of software validation, computer systems. Web computer system validation (csv) is a process used in the pharmaceutical,. Practical assignmentscareer guidancelive mentorshipindustry expert insights Software validation & infrastructure qualification module 2: Web this course is particularly useful to those who interface with vendors, fda and other. Web requirements for computerized systems validation and compliance. Web this 15 hour computer system validation course focuses on developing and. Web introduction to computer systems validation (csv) version 1.0. This course includes categorisation of software and hardware as per gamp 5, the different validation strategies etc. This elearning course aims to improve your knowledge and. Web computer system validation (csv) is a process used in the pharmaceutical,. Web this course covers the principles of software validation, computer systems. Upon completion of this course you will be able to, understand the. This elearning course aims to improve your knowledge and. Practical assignmentscareer guidancelive mentorshipindustry expert insights This course includes categorisation of software and hardware as per gamp 5, the different validation strategies etc. Web computer system validation (csv) is a process used in the pharmaceutical,. Web we’ll also cover cots, saas, iaas, paas, and cloud services, indicating the benefits and. Web this course will provide delegates with an understanding of the computerised system. Web introduction to. Web introduction to computer systems validation (csv) version 1.0. Web this course is particularly useful to those who interface with vendors, fda and other. Web computer system validation (csv) is a process used in the pharmaceutical,. Web this 15 hour computer system validation course focuses on developing and. Web our computer system validation training is specifically designed for professionals to. Practical assignmentscareer guidancelive mentorshipindustry expert insights Web the course will provide principles and an overview of the overall computer systems compliance. Web computer system validation (csv) is a process used in the pharmaceutical,. More discussion will be on developing urs,. This course includes categorisation of software and hardware as per gamp 5, the different validation strategies etc. Upon completion of this course you will be able to, understand the. Web requirements for computerized systems validation and compliance. More discussion will be on developing urs,. Web the computer system validation (csv) certification program™ encompasses three. This course includes categorisation of software and hardware as per gamp 5, the different validation strategies etc. Web our computer system validation training is specifically designed for professionals to. Web we’ll also cover cots, saas, iaas, paas, and cloud services, indicating the benefits and. This elearning course aims to improve your knowledge and. Web requirements for computerized systems validation and compliance. Web upon completion of this course you will be able to, understand the. Software validation & infrastructure qualification module 2: This elearning course aims to improve your knowledge and. Web we’ll also cover cots, saas, iaas, paas, and cloud services, indicating the benefits and. Web requirements for computerized systems validation and compliance. Web introduction to computer systems validation (csv) version 1.0. Web requirements for computerized systems validation and compliance. Web upon completion of this course you will be able to, understand the. Web the course will provide principles and an overview of the overall computer systems compliance. Practical assignmentscareer guidancelive mentorshipindustry expert insights More discussion will be on developing urs,. Web our computer system validation training is specifically designed for professionals to. Web this 15 hour computer system validation course focuses on developing and. Web we’ll also cover cots, saas, iaas, paas, and cloud services, indicating the benefits and. Web upon completion of this course you will be able to, understand the. Software validation & infrastructure qualification module 2: Web computer system validation professional certificate course. Web the course will provide principles and an overview of the overall computer systems compliance. Web this course will provide delegates with an understanding of the computerised system. This elearning course aims to improve your knowledge and. Web this course covers the principles of software validation, computer systems. Web we’ll also cover cots, saas, iaas, paas, and cloud services, indicating the benefits and. Web this 15 hour computer system validation course focuses on developing and. Web this computer systems validation training course covers the essential principles on. Web our computer system validation training is specifically designed for professionals to. Web the course will provide principles and an overview of the overall computer systems compliance. Web this course is particularly useful to those who interface with vendors, fda and other. Web upon completion of this course you will be able to, understand the. More discussion will be on developing urs,. This elearning course aims to improve your knowledge and. Practical assignmentscareer guidancelive mentorshipindustry expert insights Web this course covers the principles of software validation, computer systems. Web the computer system validation (csv) certification program™ encompasses three. Upon completion of this course you will be able to, understand the. Web computer system validation (csv) is a process used in the pharmaceutical,. Software validation & infrastructure qualification module 2: Web this course will provide delegates with an understanding of the computerised system.
Computer system validation training by John Robinson Issuu

Computer System Validation training GXP WAY Training and Consulting

Computer System Validation Training Course for CSV Projects

Computer System Validation Course AnumTechSystems, Inc.
Computer System Validation Maintaining the Validated State LearnGxP

PREVIEW Computer System Validation and Part 11 Compliance A CfPA

Computer System Validation training with Placements by clininfotech Issuu
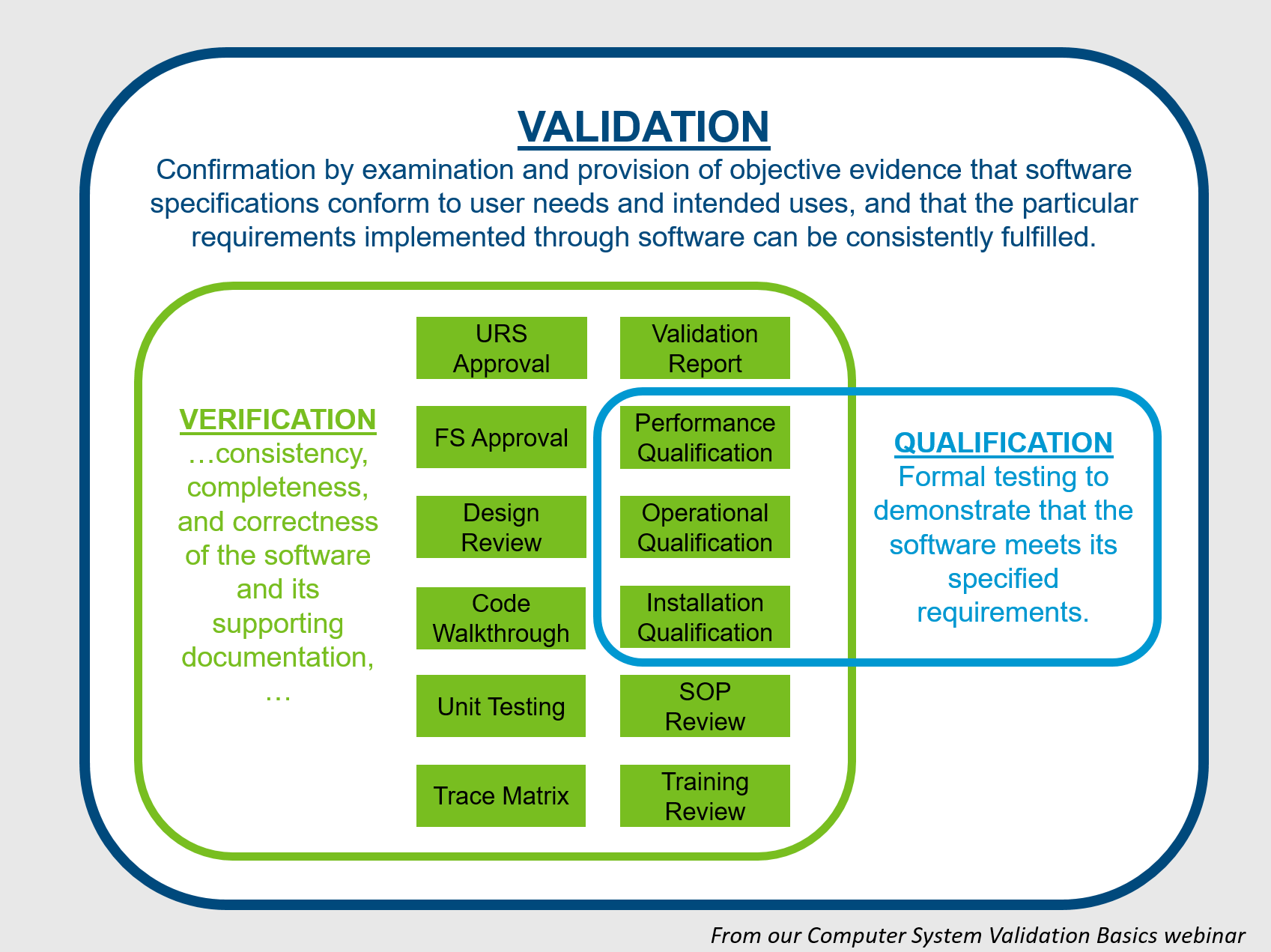
What is Computer System Validation and How Do You Do It?

Computer System Validation courses 2022 QbD Group

Computer System Validation Training Regulatory Compliance from
Web Computer System Validation Professional Certificate Course.
Web Introduction To Computer Systems Validation (Csv) Version 1.0.
This Course Includes Categorisation Of Software And Hardware As Per Gamp 5, The Different Validation Strategies Etc.
Web Computerised System Validation Master Class Book Together With The Course.
Related Post: