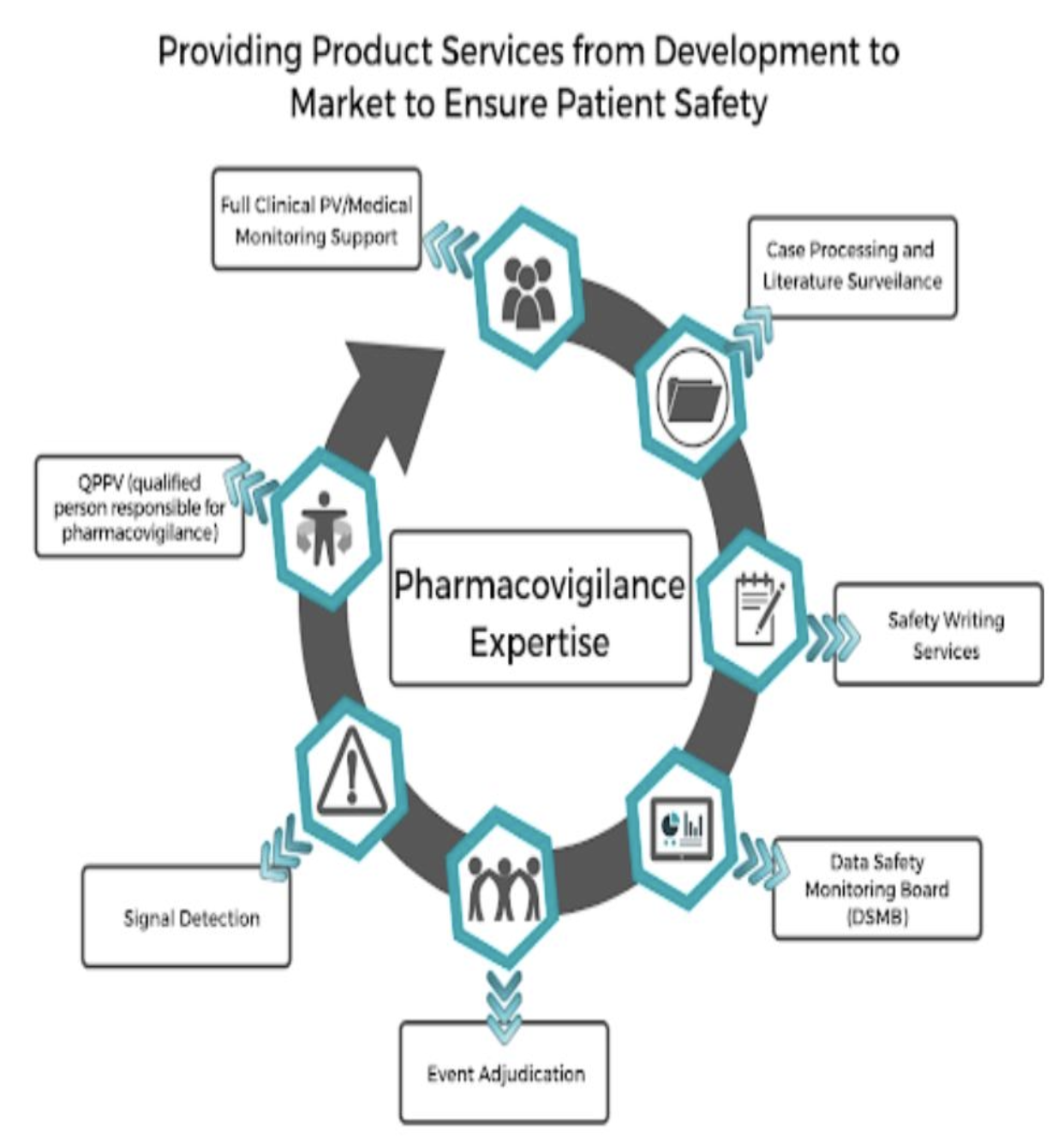
Pharmacovigilance Certificate Course
Pharmacovigilance Certificate Course - Be knowledgeable in laws, regulations, and guidelines related to drug safety and. Strengthen your knowledge in safety and pharmacovigilance by enrolling in dia's comprehensive certificate program. Web this comprehensive program is based on the dia safety and pharmacovigilance competency framework developed with experts working in the field. Professor robinson is the main instructor for this course. Some certificates can be applied toward a degree program, getting you closer to graduation while. Illinois offers 85 online certificate programs, and the list is growing. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with us and eu regulations. From topics such as collecting adr reports to data management systems and communication in pharmacovigilance, these courses will equip you with essential knowledge for your pharmacovigilance work. Web it also reviews the role monitors play in safety reporting to regulatory agencies and institutional review boards. Pharmacovigilance training brings increased employment opportunities with. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical. 23, and registration is open through oct. The fall 2023 cohort begins on oct. Web the certificate can now be completed fully online. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and. Web introductory courses in pharmacovigilance. 23, and registration is open through oct. Strengthen your knowledge in safety and pharmacovigilance by enrolling in dia's comprehensive certificate program. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with us and eu regulations. Web this innovative program prepares the student for working in the highly. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical. 1) introduction to drug safety and pharmacovigilance; Strengthen your knowledge in safety and pharmacovigilance by enrolling in dia's comprehensive certificate program. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with. Web dia’s safety and pharmacovigilance certificate program is a comprehensive program based on the dia safety and pharmacovigilance competency framework developed with experts working in the field. The fall 2023 cohort begins on oct. Pharmacovigilance training brings increased employment opportunities with. Web pharmacovigilance certification is a specialized certification program that provides individuals with knowledge and skills in the area of. Web the certificate can now be completed fully online. Educational objectives and competencies upon completion of the core courses required for the pharmacoepidemiology certificate, students will have specialized knowledge of the tools and content of pharmacoepidemiology and drug safety. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical.. 23, and registration is open through oct. Learn more and apply today. Web the medical product safety and pharmacovigilance curriculum prepares individuals to work with pharmaceutical, biologic, and medical device companies to monitor, track, and report product safety data during ongoing clinical trials, as well as after a product has been approved and marketed. Web safety and pharmacovigilance certificate program.. Web dive into the critical realm of pharmacovigilance (pv) with our comprehensive course. Educational objectives and competencies upon completion of the core courses required for the pharmacoepidemiology certificate, students will have specialized knowledge of the tools and content of pharmacoepidemiology and drug safety. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public. 3) risk management planning for medicinal products; Web the certificate can now be completed fully online. 1) introduction to drug safety and pharmacovigilance; Web introductory courses in pharmacovigilance. Web dive into the critical realm of pharmacovigilance (pv) with our comprehensive course. Pharmacovigilance training brings increased employment opportunities with. The fall 2023 cohort begins on oct. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical. Web dive into the critical realm of pharmacovigilance (pv) with our comprehensive course. Web by taking this course, you will discover how scientists are deciphering. Web learn online and earn valuable credentials from top universities like yale, michigan, stanford, and leading companies like google and ibm. This course is suitable for the candidates who wish to join any pharmaceutical industry or pursue research in the future. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences,. Educational objectives and competencies upon completion of the core courses required for the pharmacoepidemiology certificate, students will have specialized knowledge of the tools and content of pharmacoepidemiology and drug safety. Some certificates can be applied toward a degree program, getting you closer to graduation while. Lastly, the course provides learners with an overview of drug safety and pharmacovigilance. Web the certificate can now be completed fully online. Professor robinson is the main instructor for this course. This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical. 4) signal detection and management in the pharmacovigilance; Web dive into the critical realm of pharmacovigilance (pv) with our comprehensive course. It is a very popular course in medical science. These competencies outline the functional knowledge and skills needed to work in safety and pharmacovigilance and comply with us and eu regulations. 3) risk management planning for medicinal products; This program is especially well suited for individuals who are planning careers in the pharmaceutical industry, public health sciences, or biomedical. Web by taking this course, you will discover how scientists are deciphering the language of genomes to learn how to develop sustainable food and fuel supplies, improve disease treatment and prevention, and protect our environment. Web the medical product safety and pharmacovigilance curriculum prepares individuals to work with pharmaceutical, biologic, and medical device companies to monitor, track, and report product safety data during ongoing clinical trials, as well as after a product has been approved and marketed. Web dia’s safety and pharmacovigilance certificate program is a comprehensive program based on the dia safety and pharmacovigilance competency framework developed with experts working in the field. 23, and registration is open through oct.
Pharmacovigilance and Drug Safety Certificate Course Overview YouTube

Pharmacovigilance Free Online Certification Course for Pharmacy

Pharmacovigilance course Online training Pharmamentors

Online Certificate course on “Pharmacovigilance and Drug Safety

Pharmacovigilance course I Udemy certificate I Excellent material YouTube

Three month online certificate course in Pharmacovigilance

Pharmacovigilance Online Certification Course — Rasayanika
Importance of a Drug Safety Pharmacovigilance Certificate Program CCRPS

PHARMACOVIGILANCE FREE ONLINE CERTIFICATE COURSE PHARMACY STUDENTS

Certificate Course in Pharmacovigilance VigiServe Foundation
Web Safety And Pharmacovigilance Certificate Program.
Pharmacovigilance Training Brings Increased Employment Opportunities With.
Web Pharmacovigilance Certification Is A Specialized Certification Program That Provides Individuals With Knowledge And Skills In The Area Of Pharmacovigilance.
Upon Completion Of The Drug Safety And Pharmacovigilance Certificate Program, Students Will Be Able To:
Related Post: